Why Bonding and IMFs Matter (and How They Make Chemistry Feel Less Like Magic)
Imagine holding a tiny universe in your hand: atoms pulling, pushing, sharing, and sometimes stealing electrons — and from those microscopic choices come the macroscopic properties you measure in the lab: whether something melts, dissolves, conducts electricity, or floats. If you’re prepping for AP Chemistry, predicting these properties from structure is one of the highest-leverage skills you can develop. It’s not memorization; it’s pattern recognition and logical reasoning.
Big Picture: From Bond Type to Bulk Behavior
Start with a simple map: the way atoms bind (bonding) and the weaker attractions between molecules (intermolecular forces, or IMFs) control how substances behave at the scale you care about. Think of chemical bonds as the architecture of a building, and IMFs as the neighborhood — both influence what the building can do.
Core categories to keep on your finger tips
- Ionic bonds: electron transfer, lattice structures, typical of metal + nonmetal.
- Covalent bonds: electron sharing, range from nonpolar to polar covalent.
- Metallic bonds: delocalized electrons, hallmark of metals and their conductivity.
- Network covalent solids: giant covalent lattices like diamond and quartz with very high melting points.
- Intermolecular forces: dispersion (London), dipole-dipole, hydrogen bonding, ion-dipole — the forces between molecules that dictate many physical properties.
Step-by-Step Prediction Strategy
When faced with an AP-style prompt asking you to predict a property from structure (for example: “Which substance has the highest boiling point?”), use a clear sequence:
1) Identify the primary bonding type
Is it an ionic crystal, a molecular compound, a metal, or a network solid? The answer shapes the baseline expectation for melting and boiling points, conductivity, and solubility.
2) Zoom into molecular polarity and IMFs
For molecular substances: determine molecular polarity (by looking at bond polarities and geometry) and then rank IMFs: hydrogen bonds > dipole-dipole > dispersion (but remember that dispersion increases with molar mass and surface area).
3) Consider structure and size
Long, flexible molecules with large surface areas have stronger dispersion forces. Small polar molecules can still have relatively low boiling points compared to big nonpolar ones.
4) Check for special cases
Network solids (like SiO2), metallic bonding, and ionic lattices often override IMF trends. Also watch for resonance stabilization and aromaticity that change bond character.
Concrete Examples and What to Watch For
Examples are where you turn rules into intuition. Below are common AP-style comparisons with quick reasoning you can practice aloud or jot as notes.
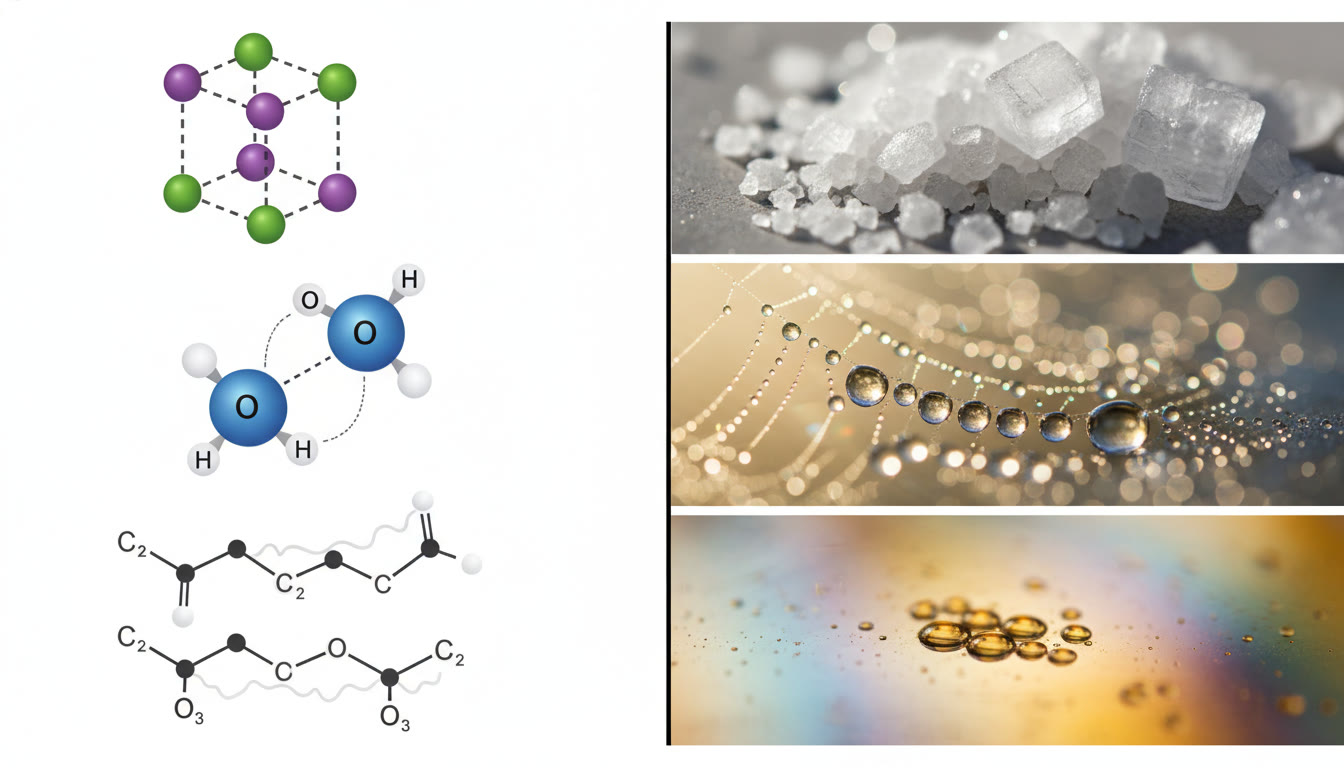
Ionic vs. Molecular: NaCl vs. H2O
Salt (NaCl) forms an ionic lattice. Water is a small polar molecule that hydrogen-bonds. Melting point: NaCl is much higher because breaking an ionic lattice costs far more energy than disrupting hydrogen bonds. Solubility: NaCl dissolves in water due to strong ion-dipole interactions; water’s hydrogen bonds also help hydrate ions. Conductivity: solid NaCl is an insulator (ions fixed in lattice), but molten or dissolved NaCl conducts; pure liquid water is a very poor conductor, while saltwater conducts well.
Dispersion dominance: I2 vs. CH3OH
Iodine (I2), although nonpolar, is a relatively heavy molecule with strong dispersion forces and is a solid at room temperature. Methanol (CH3OH) is polar and hydrogen bonds but has low molar mass so it remains a liquid. Here the mass and polarizability of iodine trump methanol’s hydrogen bonds for phase at room temperature.
Hydrogen bonding golden rule
If a molecule has N–H, O–H, or F–H and the hydrogen is bonded to a small, highly electronegative atom (N, O, or F), strong hydrogen bonding can dramatically raise boiling point and solubility in water. But don’t forget geometry — intramolecular H-bonds can reduce intermolecular H-bonding and lower expected boiling points.
Table: Quick Property Predictions from Structure
Use this table as a rapid-reference cheat sheet when deducing properties in a timed exam setting.
| Structure Type | Melting/Boiling Point | Electrical Conductivity | Solubility in Water | Representative Example |
|---|---|---|---|---|
| Ionic Lattice | Very high (strong lattice energy) | No as solid; Yes when molten or aqueous | Usually high if ions are small/solvated | NaCl |
| Polar Molecular (H-bonding) | Moderate to high (H-bonds) | Poor (unless ionized) | Often high | H2O, NH3, CH3OH |
| Nonpolar Molecular (Dispersion) | Low to moderate; increases with molar mass | Poor | Low (doesn’t dissolve well in water) | CH4, I2, C6H6 |
| Metallic | Varies; many moderate to high | Excellent (delocalized electrons) | Generally insoluble in water | Fe, Cu |
| Network Covalent | Extremely high (very strong covalent network) | Usually poor (exceptions: graphite conducts) | Generally insoluble | Diamond, SiO2 |
Practice Comparison: Which Has Higher Boiling Point?
Example prompt: Rank these by increasing boiling point — CH4, H2O, C3H8, C6H14.
Start by identifying type and size:
- CH4 (methane): nonpolar, very low molar mass — very low BP.
- C3H8 (propane): nonpolar, larger than methane — higher BP than CH4 but still low.
- C6H14 (hexane): nonpolar, much larger surface area — considerable dispersion forces — higher BP.
- H2O: polar, strong H-bonding — despite its small size, H2O has a high BP relative to similar-mass nonpolar molecules.
So the correct order (increasing BP) is: CH4 < C3H8 < C6H14 < H2O. Students who memorize this pattern internalize the power of H-bonding and polar interactions versus mere molar mass.
Common Traps and How to Avoid Them
AP questions are designed to probe understanding more than recall. Here are pitfalls students often fall into, and tips to dodge them.
Trap 1: Confusing bond polarity with molecular polarity
Two polar bonds can cancel in a symmetric shape. For example, CO2 has polar C=O bonds but is linear and nonpolar overall. Always draw the geometry, or visualize it with a quick scratch Lewis/VSEPR sketch.
Trap 2: Automatically assuming hydrogen equals strongest
Hydrogen bonding requires the hydrogen to be bonded to N, O, or F, and able to form intermolecular H-bonds. An intramolecular hydrogen bond (within the same molecule) often reduces intermolecular H-bonding and lowers boiling point.
Trap 3: Ignoring ion size and charge density
Ionic lattice energy depends on charge and radius. MgO (Mg2+ and O2-) has a much higher lattice energy and melting point than NaCl. Look at ionic charges — higher charges usually mean stronger ionic bonding.
Applying This to Lab Observations
AP free-response questions often present experimental data: conductivity tests, solubility trials, and melting point ranges. Use those pieces as clues to back-infer structure:
- If a solid conducts when melted but not as a solid → likely ionic.
- If a liquid conducts poorly and is immiscible with water → likely nonpolar molecular.
- If a material has extremely high melting point and is poor conductor → network covalent is a good candidate.
Study Techniques That Stick (and Help on Test Day)
Active practice beats passive review. Here are practical study rituals that translate into better AP performance.
1) Rapid comparisons
Create flashcards that show two or three compounds and ask you to rank by boiling point, solubility, or conductivity. Time yourself. Immediate feedback solidifies reasoning patterns.
2) Sketch-and-explain
For every molecule you study, draw the Lewis structure, VSEPR shape, and mark polar bonds. Then explain, in one sentence, why the compound has the property it does. This trains concise justification — helpful for FRQs.
3) Data-to-structure practice
Practice with mock lab data: take a single set of observations and deduce whether the substance is ionic, polar molecular, or nonpolar molecular. This mirrors the experimental framing used in AP prompts.
4) Seek targeted help
One-on-one guidance can speed breakthroughs when you’re stuck on reasoning patterns. Services like Sparkl’s personalized tutoring offer tailored study plans and expert tutors who help diagnose misunderstandings and provide AI-driven insights to optimize practice sessions — use that kind of support when you want focused improvement without wasting time.
Sample Exam-Style Prompt and Walkthrough
Prompt: Students are given four white, crystalline solids (A–D). Observations: A dissolves in water and conducts electricity when dissolved; B melts at very high temperature and does not conduct when molten; C is insoluble in water and melts at a relatively low temperature; D dissolves in water but solution is nonconductive. Identify likely bonding and justify for each.
Walkthrough
- A: Dissolves and the solution conducts → ions free to move → ionic compound.
- B: Very high melting point and does not conduct when molten → cannot be ionic; likely a network covalent solid (e.g., quartz) or a covalent solid with a huge lattice.
- C: Insoluble and low melting point → nonpolar molecular substance with weak dispersion forces.
- D: Dissolves but the solution is nonconductive → likely covalent but polar and molecular (it dissolves by hydrogen bonding or dipole interactions) or a molecular acid that does not ionize significantly; check for ionization to decide.
Real-World Context: Why This Skill Matters Beyond the Exam
Predicting material properties from bonding and structure is not just an AP exercise — it’s foundational to materials science, pharmaceuticals, environmental chemistry, and even engineering. When you can look at a molecular formula and infer reactivity, stability, and macroscopic behavior, you’re thinking like a chemist and unlocking the applied side of science: why polymers behave as they do, how drugs dissolve, and why desalination requires specific ionic manipulations.
Troubleshooting Tough Questions: A Short Checklist
When you freeze on an AP problem, run through this checklist quickly:
- What is the bonding type? Ionic, covalent, metallic, or network?
- Is the molecule polar? Are there hydrogen bond donors/acceptors?
- How large is the molecule? Does surface area suggest big dispersion forces?
- Are experimental clues given? Conductivity, solubility, phase at room temperature?
- Any special structural features: resonance, conjugation, ring strain, aromaticity?
Final Advice for Exam Day
Keep your answers structured, concise, and justified. For free-response questions, give one or two sentences of reasoning that explicitly tie structure to property: e.g., “Compound X has hydrogen-bond donors (O–H) and is small, so extensive hydrogen bonding raises its boiling point relative to nonpolar molecules of similar mass.” That shows command of cause-and-effect — exactly what AP graders look for.
Closing: Make Structure Your Superpower
Mastering chemical bonding and intermolecular forces turns vague facts into a powerful prediction engine. With focused practice — rapid comparisons, sketch-and-explain drills, and data-to-structure challenges — you’ll move from memorizing to reasoning. If you want targeted, personalized help, a few sessions with a skilled tutor or a structured program like Sparkl’s personalized tutoring can compress months of learning into weeks by diagnosing misconceptions and tailoring practice. But at the core, consistent active practice and thinking like a chemist will carry you through the AP exam and beyond.
Start small: pick three molecules right now, predict three properties for each, then check your reasoning. The habit of predicting, justifying, and reflecting will make structure your superpower — and that’s the real win.

















No Comments
Leave a comment Cancel